Role of T-cell receptors
T-cell receptor (TCR) T-cell therapy design1
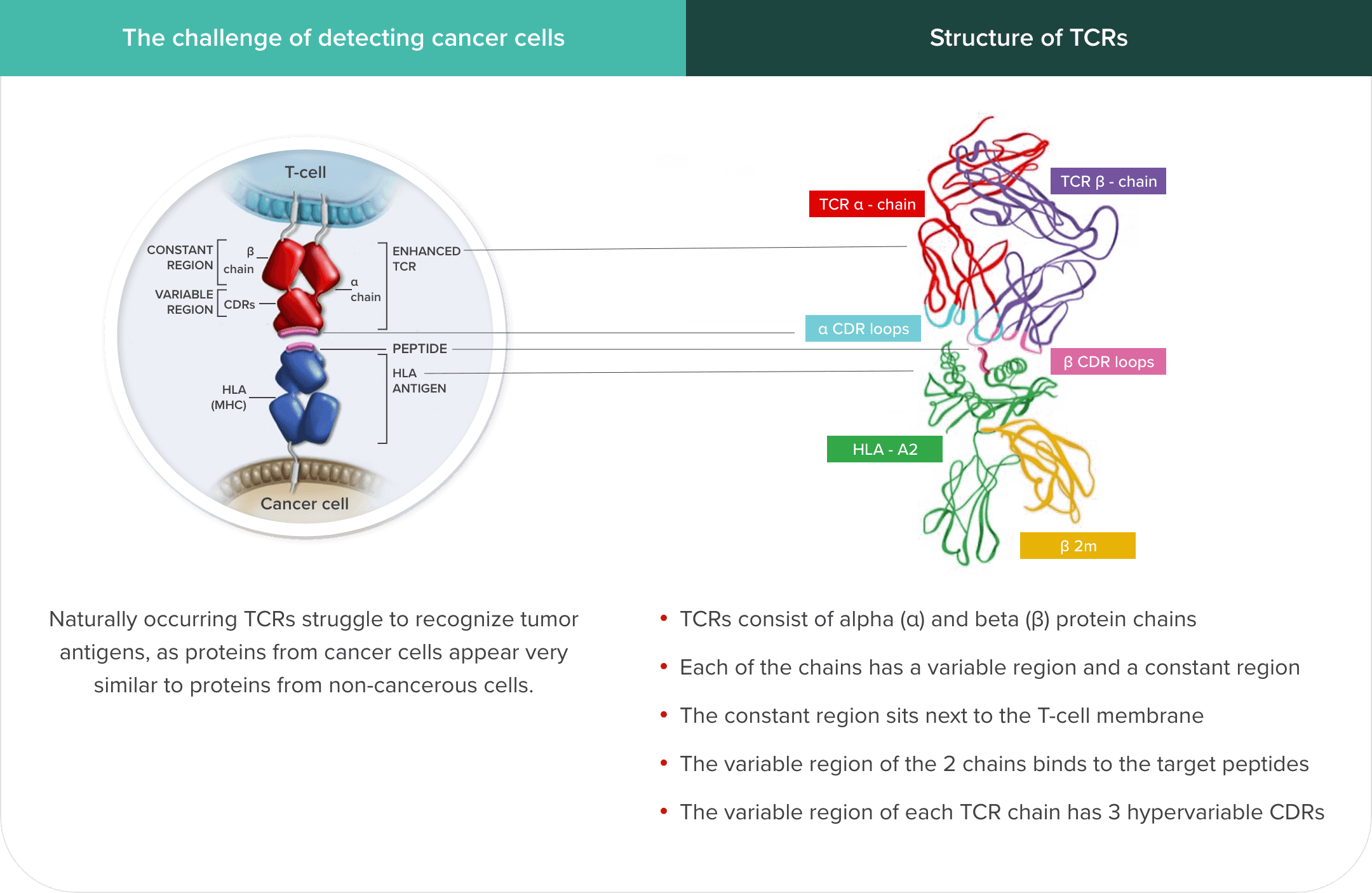
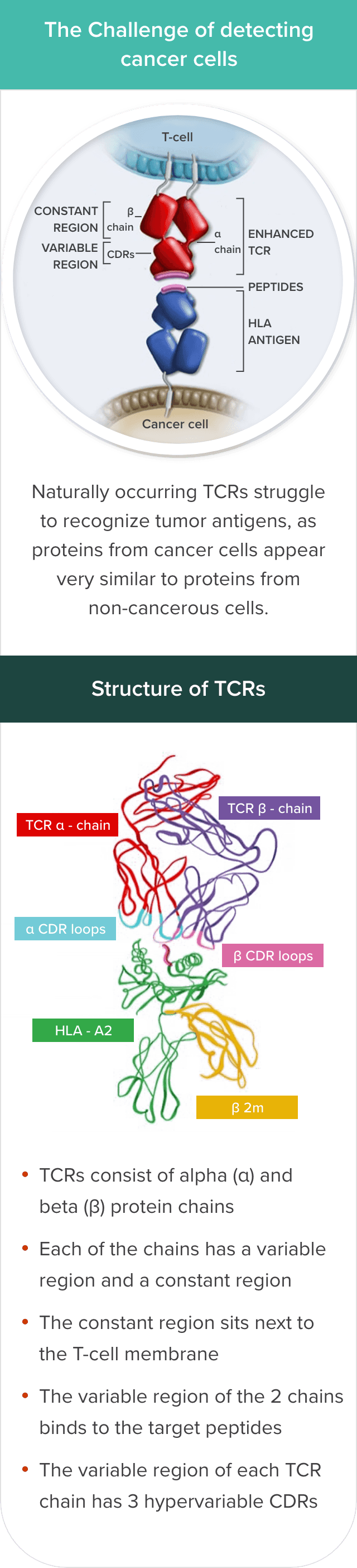
T cells’ ability to recognize and infiltrate tumor cells may be limited by several factors, including reduced tumor antigen expression by tumor cells, a scarcity of high-affinity, tumor-reactive T cells, and immunosuppressive factors associated with the tumor and microenvironment.2,3
TCR T-cell therapies are designed to: 1) leverage genetic modification with a lentivirus vector encoding a TCR that has increased affinity for human leukocyte antigen (HLA)–tumor antigen complexes in cancer cells with the target antigen; 2) enable the infusion of a large number of engineered T cells that may induce the adaptive immune response, binding to and killing tumor cells; and, 3) work with lymphodepleting chemotherapy to optimize T-cell persistence.2,4-7
Genetically modified TCRs have increased affinity for HLA–tumor antigen complexes but keep a very similar structure to endogenous TCRs8
Michaela Sharpe, Natalie Mount, Genetically modified T cells in cancer therapy: opportunities and challenges, Dis Model Mech, 2015, Fig. 3., Copyright 2024 The Company of Biologists
The HLA–tumor antigen peptide complex provides specificity to the target-binding process.6,9
However, identifying suitable target antigens has been a major challenge in the development of TCR T-cell therapies for solid tumors. The characteristics of an ideal antigen for effective targeting include selective expression on tumor cells, including expression of intracellular antigens on the cell surface as an HLA–tumor antigen peptide complex, and no or low expression on healthy cells.3,10 The ideal antigen should also have sufficient immunogenicity to mediate cytotoxic T-cell responses and play a key role in tumor cell survival.3 Cancer/testis antigens (CTAs) meet most of these criteria.
CTAs:
- Are a type of intracellular tumor antigen.11,12 Tumor cells process intracellular antigens in the endoplasmic reticulum and express them as a peptide complex with HLA on the surface of tumor cells that can be targeted to stimulate an antitumor immune response10
- Are not usually expressed on somatic tissues13
- Are only expressed on male germ cells in healthy adults; however, these cells lack HLA expression and do not present antigens to T cells13
- May be highly immunogenic, including New York esophageal squamous cell carcinoma 1 (NY-ESO-1), with many patients having antibodies and reactive T cells that are unable to stop the cancer from growing14,15
- Are reexpressed during the dedifferentiation process in carcinogenesis in many types of cancers16-18
Examples of CTAs under investigation include19-23:
- Melanoma-associated antigen A4 (MAGE-A4)
- NY-ESO-1
- Preferentially expressed antigen in melanoma (PRAME)
- Synovial sarcoma X (SSX)
- P antigen family member 5 (PAGE5)
- Melanoma antigen recognized by T cells 1 (MART-1)
- Glycoprotein 100 (GP100)
TCRs recognize and bind to HLA–tumor antigen peptide complexes6
HLAs play an important role in regulating the immune system. They present peptide/antigen complexes to TCRs, enabling the immune system to differentiate between self and nonself.24
TCR T cells can target both membrane-bound extracellular proteins and intracellular proteins that are displayed as peptides complexed with their specific HLA on the surface of tumor cells.6,9
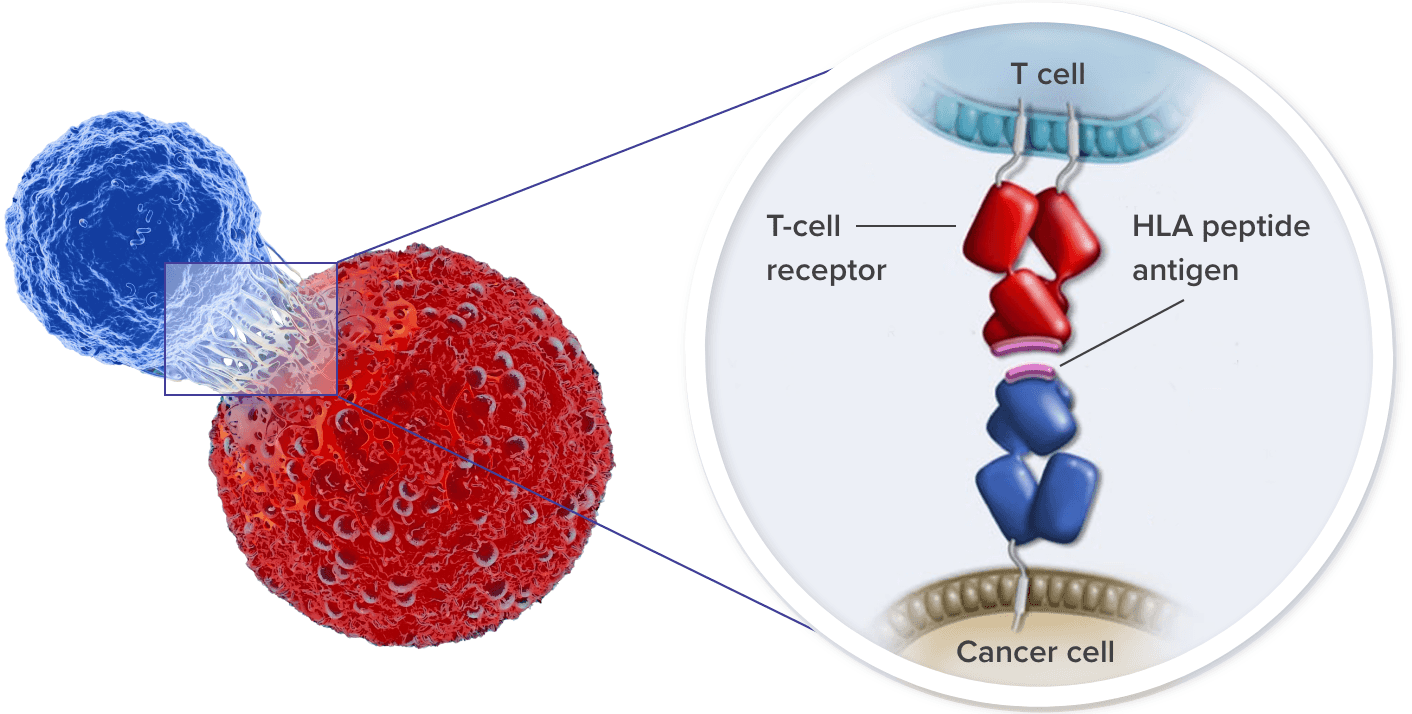
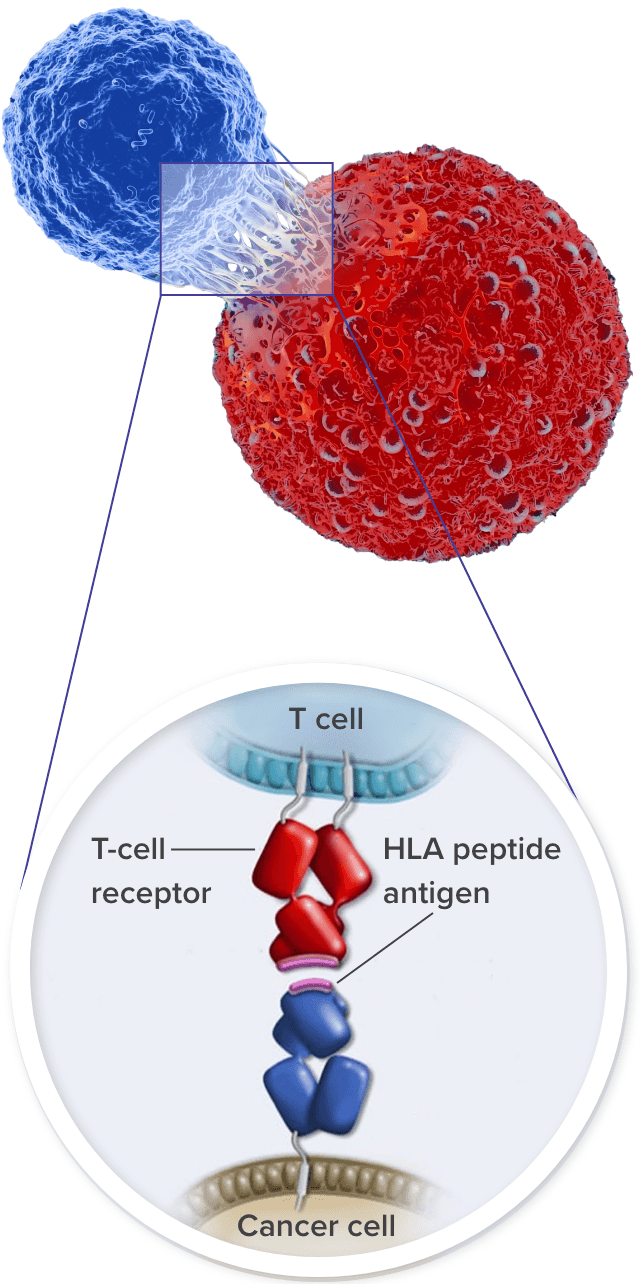
T cells binding to the tumor via TCRs activate a signaling cascade to induce tumor cell killing.6,25
- Cytotoxic (CD8+) T cells kill tumor cells via the release of granzymes and perforin26
- These cells also produce interferon-γ (IFNγ) to increase the expression of HLA by tumor cells, making them better targets for CD8+ T cells27,28
T-cell therapies are designed to localize when and where the therapeutic and toxic action of T cells occurs.29
CAR=chimeric antigen receptor; CD=cluster of differentiation; CDR=complementarity-determining region; MHC=major histocompatibility complex; scFv=single-chain variable fragment; TNFr=tumor necrosis factor receptor.
Next
Role of biomarkers in T-cell receptor T-cell therapies
References
- Adaptimmune Therapeutics plc. Technology. Accessed September 27, 2023. https://www.adaptimmune.com/technology
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1–specific TCR–engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914-921.
- Rath JA, Arber C. Engineering strategies to enhance TCR-based adoptive T cell therapy. Cells. 2020;9(6):1485.
- Baruch EN, Berg AL, Besser MJ, Schachter J, Markel G. Adoptive T cell therapy: an overview of obstacles and opportunities. Cancer. 2017;123(S11):2154-2162.
- Tsimberidou AM, Van Morris K, Vo HH, et al. T-cell receptor-based therapy: an innovative therapeutic approach for solid tumors. J Hematol Oncol. 2021;14(1):102.
- Zhang J, Wang L. The emerging world of TCR-T cell trials against cancer: a systematic review. Technol Cancer Res Treat. 2019;18:1533033819831068.
- Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474(4):449-461.
- Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8(4):337-350.
- Zhao Q, Jiang Y, Xiang S, et al. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol. 2021;12:658753.
- He Q, Jiang X, Zhou X, Weng J. Targeting cancers through TCR-peptide/MHC interactions. J Hematol Oncol. 2019;12(1):139.
- Yarchoan M, Johnson BA III, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209-222.
- Al-Khadairi G, Decock J. Cancer testis antigens and immunotherapy: where do we stand in the targeting of PRAME? Cancers (Basel). 2019;11(7):984.
- Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6(18):15772-15787.
- Thomas R, Al-Khadairi G, Roelands J, et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947.
- Jäger E, Stockert E, Zidianakis Z, et al. Humoral immune responses of cancer patients against “cancer-testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84(5):506-510.
- Kim JJ, Rajagopalan K, Hussain B, Williams BH, Kulkarni P, Mooney SM. CETN1 is a cancer testis antigen with expression in prostate and pancreatic cancers. Biomark Res. 2013;1(1):22.
- Shuvalov O, Kizenko A, Petukhov A, Fedorova O, Daks A, Barlev N. Emerging roles of cancer-testis antigenes, semenogelin 1 and 2, in neoplastic cells. Cell Death Discov. 2021;7(1):97.
- Bode PK, Thielken A, Brandt S, et al. Cancer testis antigen expression in testicular germ cell tumorigenesis. Mod Pathol. 2014;27(6):899-905.
- Wang M, Li J, Wang L, et al. Combined cancer testis antigens enhanced prediction accuracy for prognosis of patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(4):3513-3528.
- Albertsmeier M, Altendorf-Hofmann A, Lindner LH, et al. Cancer testis antigens and immunotherapy: expression of PRAME is associated with prognosis in soft tissue sarcoma. Cancers. 2020;12(12):3612.
- Li XF, Ren P, Shen WZ, Jin X, Zhang J. The expression, modulation and use of cancer-testis antigens as potential biomarkers for cancer immunotherapy. Am J Transl Res. 2020;12(11):7002-7019.
- Chen YT, Stockert E, Jungbluth A, et al. Serological analysis of Melan-A(MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Natl Acad Sci U S A. 1996;93(12):5915-5919.
- Martinez-Perez D, Viñal D, Solares I, Espinosa E, Feliu J. Gp-100 as a novel therapeutic target in uveal melanoma. Cancers (Basel). 2021;13(23):5968.
- Crux NB, Elahi S. Human leukocyte antigen (HLA) and immune regulation: how do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections? Front Immunol. 2017;8:832.
- Knapp B, van der Merwe PA, Dushek O, Deane CM. MHC binding affects the dynamics of different T-cell receptors in different ways. PLoS Comput Biol. 2019;15(9):e1007338.
- Santos-Zas I, Lemarié J, Zlatanova I, et al. Cytotoxic CD8+ T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat Commun. 2021;12(1):1483.
- Zhang S, Kohli K, Black RG, et al. Systemic interferon-Y increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol Res. 2019;7(8):1237-1243.
- Tsukumo SI, Yasutomo K. Regulation of CD8+ T cells and antitumor immunity by Notch signaling. Front Immunol. 2018;9:101.
- Roybal KT, Rupp LJ, Morsut L, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770-779.